During lab, you measured the heat of reaction by mixing solutions of FeCl3 and NaOH.
(a) Writ...

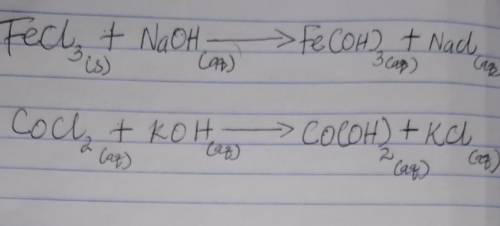
During lab, you measured the heat of reaction by mixing solutions of FeCl3 and NaOH.
(a) Write the balanced net precipitation reaction that occurred. (Use the lowest possible coefficients. Include states-of-matter under the given conditions in your answer.)
(b) The experiment could also be done with solutions of CoCl2 and KOH. What is the balanced net precipitation reaction for this mixture?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3


Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 23.06.2019 03:30
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1
You know the right answer?
Questions


Mathematics, 16.09.2019 14:00




English, 16.09.2019 14:00

Advanced Placement (AP), 16.09.2019 14:00

Business, 16.09.2019 14:00

Geography, 16.09.2019 14:00

Spanish, 16.09.2019 14:00

World Languages, 16.09.2019 14:00



Mathematics, 16.09.2019 14:00


Biology, 16.09.2019 14:00

Mathematics, 16.09.2019 14:00

English, 16.09.2019 14:00

Mathematics, 16.09.2019 14:00

Mathematics, 16.09.2019 14:00