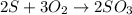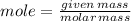Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1


Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2

Chemistry, 23.06.2019 05:00
How is electrolysis most commonly used to produce an energy source? a - splitting water molecules produces oxygen, which organisms breathe to fuel their bodies. b - splitting water molecules produces hydrogen gas, which is used to power machines through hydrogen fuel cells. c - splitting carbon dioxide molecules produces coal, a form of carbon that can be burned to produce heat. d - splitting carbon dioxide molecules produces natural gas, which can be burned to generate electricity in power plants.
Answers: 1
You know the right answer?
60.0 g O2 and 50.0 g S are reacted according to the equation 2 S + 3 O2 → 2 SO3 . Which reactant is...
Questions

Mathematics, 13.07.2019 04:30

Mathematics, 13.07.2019 04:30

Geography, 13.07.2019 04:30

Mathematics, 13.07.2019 04:30


Social Studies, 13.07.2019 04:30

Mathematics, 13.07.2019 04:30

Chemistry, 13.07.2019 04:30

Mathematics, 13.07.2019 04:30

Social Studies, 13.07.2019 04:30




Mathematics, 13.07.2019 04:30

History, 13.07.2019 04:30

Chemistry, 13.07.2019 04:30

Mathematics, 13.07.2019 04:30

Mathematics, 13.07.2019 04:30

Mathematics, 13.07.2019 04:30

Mathematics, 13.07.2019 04:30