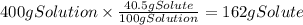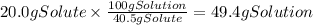Chemistry, 14.04.2020 22:30 hannahblank2466
A chemistry student needs of acetic acid for an experiment. He has available of a w/w solution of acetic acid in acetone. Calculate the mass of solution the student should use. If there's not enough solution, press the "No solution" button. Round your answer to significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
A chemistry student needs of acetic acid for an experiment. He has available of a w/w solution of ac...
Questions

History, 29.09.2019 13:00



Mathematics, 29.09.2019 13:00

History, 29.09.2019 13:00

Social Studies, 29.09.2019 13:00



Biology, 29.09.2019 13:00


Biology, 29.09.2019 13:00

English, 29.09.2019 13:00


History, 29.09.2019 13:00

Biology, 29.09.2019 13:00

Mathematics, 29.09.2019 13:00

Mathematics, 29.09.2019 13:00