

Answers: 3
Another question on Chemistry


Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1
You know the right answer?
Lithium and nitrogen react in a combination reaction to produce lithium nitride: 6Li(s) + N2(g) → 2L...
Questions



Mathematics, 19.10.2019 04:30


Chemistry, 19.10.2019 04:30

Mathematics, 19.10.2019 04:30



Mathematics, 19.10.2019 04:30


History, 19.10.2019 04:30

Social Studies, 19.10.2019 04:30




Biology, 19.10.2019 04:30


Geography, 19.10.2019 04:30

Mathematics, 19.10.2019 04:30

Chemistry, 19.10.2019 04:30

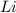 are needed to produce 0.45 mol of
are needed to produce 0.45 mol of 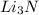
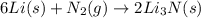
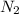 is the excess reagent.
is the excess reagent.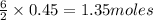 of
of 

