
Chemistry, 14.04.2020 23:47 sgslayerkingminecraf
The rate of a hypothetical reaction involving L and M is found to double when the concentration of L is doubled and to increase fourfold when the concentration of M is doubled. Write the rate law for this reaction.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:00
If a reaction has g of -136kj at 110°c, will it be spontaneous at this temperature (110°c)? yes or no
Answers: 2

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3


Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2
You know the right answer?
The rate of a hypothetical reaction involving L and M is found to double when the concentration of L...
Questions

History, 17.03.2020 04:27



Biology, 17.03.2020 04:27

Biology, 17.03.2020 04:27

Mathematics, 17.03.2020 04:27


Mathematics, 17.03.2020 04:27


Mathematics, 17.03.2020 04:28



Computers and Technology, 17.03.2020 04:28


Mathematics, 17.03.2020 04:28

Mathematics, 17.03.2020 04:28

Mathematics, 17.03.2020 04:28


Biology, 17.03.2020 04:28

![Rate=k[L]^1[M]^2](/tpl/images/0600/0548/edc82.png)
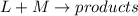
![Rate=k[L]^x[M]^y](/tpl/images/0600/0548/8fe10.png) (1)
(1)![2\times Rate=k[2L]^x[M]^y](/tpl/images/0600/0548/f32b0.png) (2)
(2)![4\times Rate=k[L]^x[2M]^y](/tpl/images/0600/0548/866b5.png) (3)
(3)![\frac{2\times Rate}{Rate}=\frac{k[2L]^x[M]^y}{k[L]^x[M]^y}](/tpl/images/0600/0548/76345.png)
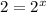
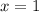
![\frac{4\times Rate}{Rate}=\frac{k[L]^x[2M]^y}{k[L]^x[M]^y}](/tpl/images/0600/0548/beaf5.png)
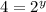
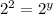
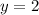
![k[L]^1[M]^2](/tpl/images/0600/0548/2ee9e.png)


