Chemistry, 15.04.2020 02:11 espinosajoselyn
A 5.91 g of octane, C8H18, reacts with excess oxygen in a bomb calorimeter. The heat capacity of the calorimeter is 6.97 kJ/°C and the temperature increases by 32.0°C. How much heat, in units of kJ/mol, was absorbed by the bomb calorimeter?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

Chemistry, 23.06.2019 06:40
8. how much enthalpy/heat is transferred when 0.5113gof ammonia (nh3) reacts with excess oxygen according| to the following equation: 4nh3 +502 - 4n0+ 6h20ah = -905.4j
Answers: 1

You know the right answer?
A 5.91 g of octane, C8H18, reacts with excess oxygen in a bomb calorimeter. The heat capacity of the...
Questions

Biology, 13.07.2019 01:00




Biology, 13.07.2019 01:00


History, 13.07.2019 01:00

Computers and Technology, 13.07.2019 01:00


English, 13.07.2019 01:00


Mathematics, 13.07.2019 01:00

Mathematics, 13.07.2019 01:00



History, 13.07.2019 01:00

Mathematics, 13.07.2019 01:00

Mathematics, 13.07.2019 01:00


Mathematics, 13.07.2019 01:00

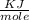
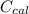 = 6.97
= 6.97 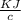
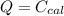 ΔT
ΔT