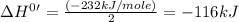Chemistry, 15.04.2020 02:09 claudia122752
The standard enthalpy change for the following reaction is 232 kJ at 298 K. 2 H2CO(g) 2 C(s, graphite) + 2 H2(g) + O2(g) ΔH° = 232 kJ What is the standard enthalpy change for this reaction at 298 K? C(s, graphite) + H2(g) + 1/2 O2(g) H2CO(g)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 23.06.2019 06:30
Which of these natural resources is non-renewable a.corn b.wind c.geothermal d.natural gas
Answers: 2

Chemistry, 23.06.2019 07:10
Which one of the following is an oxidation-reduction reaction? naoh + hno3 --> h2o + kno3 naoh + hno3 --> h2o + kno3 so3 + h2o --> h2so4 cacl2 + na2co3 --> caco3 + 2 nacl ch4 + 2 o2 --> co2 + 2 h2o al2(so4)3 + 6 koh --> 2 al(oh)3 + 3 k2so4
Answers: 3

You know the right answer?
The standard enthalpy change for the following reaction is 232 kJ at 298 K. 2 H2CO(g) 2 C(s, graphit...
Questions


Mathematics, 25.02.2021 14:00



French, 25.02.2021 14:00


Mathematics, 25.02.2021 14:00


English, 25.02.2021 14:00


Physics, 25.02.2021 14:00

History, 25.02.2021 14:00







Mathematics, 25.02.2021 14:00

Computers and Technology, 25.02.2021 14:00

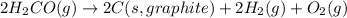
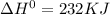
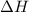 for the following reaction i.e,
for the following reaction i.e,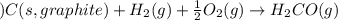

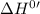 for the reaction will be:
for the reaction will be: