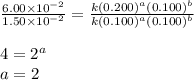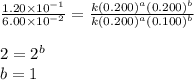Chemistry, 15.04.2020 01:53 beautycutieforever10
For the reaction 3A(g) + 2B(g) → 2C(g) + 2D(g) the following data were collected at constant temperature. Determine the correct rate law for this reaction. Trial Initial [A] Initial [B] Initial Rate (mol/L) (mol/L) (mol/(L·min)) 1 0.200 0.100 6.00 × 10–2 2 0.100 0.100 1.50 × 10–2 3 0.200 0.200 1.20 × 10–1 4 0.300 0.200 2.70 × 10–1

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:00
The overall chemical reaction for photosynthesis isshown below: 6co2 + 6h20 → c6h12o6 + 602what mass of glucose (c6h1206) can form from71.89 g co2? (molar mass of c6h1206 = 180.18g/mol; molar mass of co2 = 44.01 g/mol)71.89 g co2=g c6h1206
Answers: 1

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3
You know the right answer?
For the reaction 3A(g) + 2B(g) → 2C(g) + 2D(g) the following data were collected at constant tempera...
Questions




Mathematics, 18.08.2021 04:00





Biology, 18.08.2021 04:00

Physics, 18.08.2021 04:00

Mathematics, 18.08.2021 04:00


Social Studies, 18.08.2021 04:00

Mathematics, 18.08.2021 04:00

English, 18.08.2021 04:00

History, 18.08.2021 04:00



Physics, 18.08.2021 04:00

History, 18.08.2021 04:00

![\text{Rate}=k[A]^2[B]^1](/tpl/images/0600/6826/858f4.png)
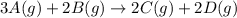
![\text{Rate}=k[A]^a[B]^b](/tpl/images/0600/6826/10aeb.png)
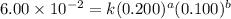 ....(1)
....(1)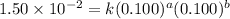 ....(2)
....(2)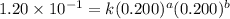 ....(3)
....(3)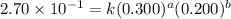 ....(4)
....(4)