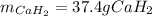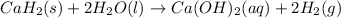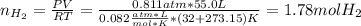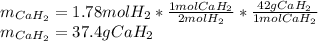Chemistry, 15.04.2020 00:28 swaggirllely36
Calcium hydride (CaH2) reacts with water to form hydrogen gas: CaH2(s) + 2 H2O(l) → Ca(OH)2(aq) + 2 H2(g) Determine the number of grams of CaH2 are needed to generate 55.0 L of H2 gas at a pressure of 0.811 atm and a temperature of 32°C.\

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1
You know the right answer?
Calcium hydride (CaH2) reacts with water to form hydrogen gas: CaH2(s) + 2 H2O(l) → Ca(OH)2(aq) + 2...
Questions


Biology, 29.06.2021 05:30


Mathematics, 29.06.2021 05:30

Chemistry, 29.06.2021 05:30



English, 29.06.2021 05:30

Chemistry, 29.06.2021 05:30

Mathematics, 29.06.2021 05:30




Mathematics, 29.06.2021 05:30

Mathematics, 29.06.2021 05:30


Mathematics, 29.06.2021 05:30

Biology, 29.06.2021 05:30

Mathematics, 29.06.2021 05:30