
Chemistry, 15.04.2020 00:47 shellxavier1
For the decomposition of phosphorous pentachloride to phosphorous trichloride and chlorine at 400K the KC is 1.1x10-2. Given that 1.0g of phosphorous pentachloride is added to a 250mL reaction flask, find the percent decomposition after the system has reached equilibrium.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1
You know the right answer?
For the decomposition of phosphorous pentachloride to phosphorous trichloride and chlorine at 400K t...
Questions

Social Studies, 25.07.2019 02:30







Geography, 25.07.2019 02:30



Biology, 25.07.2019 02:30





Mathematics, 25.07.2019 02:30

English, 25.07.2019 02:30

Mathematics, 25.07.2019 02:30

Mathematics, 25.07.2019 02:30

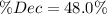
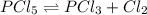
![[PCl_5]_0=\frac{1.0g*\frac{1mol}{208.24g} }{0.250L} =0.0192M](/tpl/images/0600/3535/2eefd.png)
 is introduced and the law of mass action is written as shown below:
is introduced and the law of mass action is written as shown below:![Kc=\frac{[PCl_3][Cl_2]}{[PCl_5]}=\frac{(x)(x)}{(0.0192-x)} =1.1x10^{-2}](/tpl/images/0600/3535/ffd3a.png)
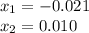
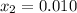 , thus, the equilibrium concentration of phosphorous pentachloride is:
, thus, the equilibrium concentration of phosphorous pentachloride is:![[PCl_5]_{eq}=0.0192M-0.01M=0.00921M](/tpl/images/0600/3535/f13fa.png)
![\% Dec=\frac{[PCl_5]_{eq}}{[PCl_5]_0}*100\% =\frac{0.00921M}{0.0192M} *100\%\\\% Dec=48.0\%](/tpl/images/0600/3535/f20dd.png)


