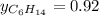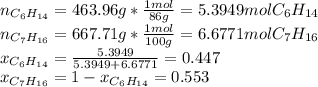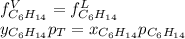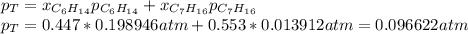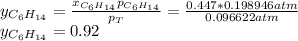Chemistry, 15.04.2020 00:51 malachilaurenc
Two linear hydrocarbons, Hexane (C6H14) and Heptane (C7H16), form pretty much an ideal solution at any composition. A solution is made at 25°C that contains 463.96 g of Hexane in 667.71 g Heptane: Characterise the vapour above this solution, and answer, What is the mole fraction of Hexane in the vapour?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3
You know the right answer?
Two linear hydrocarbons, Hexane (C6H14) and Heptane (C7H16), form pretty much an ideal solution at a...
Questions


English, 11.03.2021 23:00

Chemistry, 11.03.2021 23:00


Mathematics, 11.03.2021 23:00

Mathematics, 11.03.2021 23:00



Mathematics, 11.03.2021 23:00


Mathematics, 11.03.2021 23:00

Mathematics, 11.03.2021 23:00

Social Studies, 11.03.2021 23:00



Mathematics, 11.03.2021 23:00

History, 11.03.2021 23:00

History, 11.03.2021 23:00

Chemistry, 11.03.2021 23:00