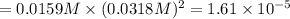PbCl2 (s) ⇆ Pb2+ (aq) + 2Cl− (aq)

Chemistry, 15.04.2020 00:52 genyjoannerubiera
Consider the dissolution equation of lead(II) chloride.
PbCl2 (s) ⇆ Pb2+ (aq) + 2Cl− (aq)
Suppose you add 0.2307 g of PbCl2 (s) to 50.0 mL of water. In the resulting saturated solution, you find that the concentration of Pb2+ (aq) is 0.0159 M and the concentration of Cl− (aq) is 0.0318 M.
What is the value of the equilibrium constant, Ksp, for the dissolution of PbCl2?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1
You know the right answer?
Consider the dissolution equation of lead(II) chloride.
PbCl2 (s) ⇆ Pb2+ (aq) + 2Cl− (aq)
PbCl2 (s) ⇆ Pb2+ (aq) + 2Cl− (aq)
Questions


Chemistry, 12.10.2020 23:01





History, 12.10.2020 23:01


Biology, 12.10.2020 23:01


Business, 12.10.2020 23:01



Mathematics, 12.10.2020 23:01

Mathematics, 12.10.2020 23:01

Physics, 12.10.2020 23:01

Biology, 12.10.2020 23:01


History, 12.10.2020 23:01

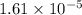 .
.![[Pb^{2+}]=0.0159 M](/tpl/images/0600/3825/89ec5.png)
![[Cl^-]=0.0318 M](/tpl/images/0600/3825/75929.png)
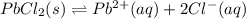
![K_{sp}=[Pb^{2+}][Cl^-]^2](/tpl/images/0600/3825/7fd11.png)