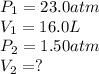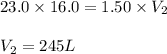Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1


Chemistry, 23.06.2019 10:30
What is the difference between skimming and absorbing methods of the oil removal
Answers: 2

Chemistry, 23.06.2019 11:50
What is the oxidation half-reaction for this unbalanced redox equation? cr2o72– + fe2+ → cr3+ + fe3+ cr3+ → cr2o72– cr2o72– → cr3+ fe3+ → fe2+ fe2+ → fe3+?
Answers: 2
You know the right answer?
A 16.0 16.0 L sample of neon gas has a pressure of 23.0 23.0 atm at a certain temperature. At the sa...
Questions

Mathematics, 09.07.2019 20:40




History, 09.07.2019 20:40


Computers and Technology, 09.07.2019 20:40



Mathematics, 09.07.2019 20:40



Mathematics, 09.07.2019 20:40


History, 09.07.2019 20:40




Mathematics, 09.07.2019 20:40

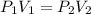
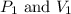 are initial pressure and volume.
are initial pressure and volume.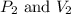 are final pressure and volume.
are final pressure and volume.