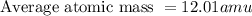Chemistry, 15.04.2020 01:49 RiceLord9562
Jmap Base your answer to the question on the information. Naturally occurring elemental carbon is a mixture of isotopes. The percent composition of the two most abundant isotopes is listed below.98.93% of the carbon atoms have a mass of 12.00 atomic mass units.1.07% of the carbon atoms have a mass of 13.00 atomic mass units. Calculate the average atomic mass of carbon.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 23.06.2019 02:00
Which statement is true about the model of the electromagnetic spectrum a: it change the frequencies of light. b: it compare wavelengths of light. c: the color of light waves can be changed using the model. d: the intensities of light waves can be decreased using the model.
Answers: 2


Chemistry, 23.06.2019 06:30
Achemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 no(g) + cl2(g) < => 2 nocl(g) kp = 2 x 10^(-6)he fills a reaction vessel at this temperature with 13. atm of nitrogen monoxide gas and 12. atm of chlorine gas. use this data to answer the questions: a. can you predict the equilibrium pressure of noci, using only the tools available to you within aleks? y/nb. if you said yes, then enter the equilibrium pressure of nocl at right. round your answer to 1 significant digit.
Answers: 1
You know the right answer?
Jmap Base your answer to the question on the information. Naturally occurring elemental carbon is a...
Questions

Mathematics, 22.02.2021 08:00

Mathematics, 22.02.2021 08:00


Mathematics, 22.02.2021 08:00

World Languages, 22.02.2021 08:00

Mathematics, 22.02.2021 08:00



Health, 22.02.2021 08:00


Mathematics, 22.02.2021 08:00


Mathematics, 22.02.2021 08:00

Mathematics, 22.02.2021 08:00



Health, 22.02.2021 08:00



Mathematics, 22.02.2021 08:00

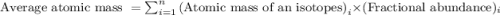
![\text{Average atomic mass }=[(12.00\times 0.9893)+(13\times 0.0107)]](/tpl/images/0600/6598/dc2f3.png)