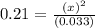Chemistry, 15.04.2020 02:43 prayingfeet7799
Consider the following reaction, equilibrium concentrations, and equilibrium constant at a particular temperature. Determine the equilibrium concentration of NO2(g). N2O4(g) ↔ 2 NO2(g) Kc = 0.21 [N2O4]eq = 0.033 M

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1

Chemistry, 23.06.2019 14:00
[08.05] which answer provides the correct name for name the following hydrocarbon? (2 points) moving left to right: a hydrocarbon chain made of a methyl group (ch subscript three) single bond carbon triple bond carbon single bond ch bonded to a methyl group (ch subscript three) and a chain including a methelyne group (ch subscript two) single bond methyl group (ch subscript three). 4-ethyl-2-pentyne 4-methyl-2-hexyne 2-heptyne 4-methy-hexane
Answers: 1

Chemistry, 23.06.2019 16:30
If 950 joules of heat are needed to increase the temperature of 45.6 grams of an unknown metal from 25.0 celsius to 90.0 celsius, what is the specific heat capacity of the metal in j/g celsius
Answers: 2
You know the right answer?
Consider the following reaction, equilibrium concentrations, and equilibrium constant at a particula...
Questions


Geography, 19.10.2020 14:01

Mathematics, 19.10.2020 14:01

Mathematics, 19.10.2020 14:01

History, 19.10.2020 14:01

Mathematics, 19.10.2020 14:01

Mathematics, 19.10.2020 14:01


Mathematics, 19.10.2020 14:01




English, 19.10.2020 14:01

Mathematics, 19.10.2020 14:01

Computers and Technology, 19.10.2020 14:01

World Languages, 19.10.2020 14:01


Chemistry, 19.10.2020 14:01

Social Studies, 19.10.2020 14:01

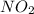 is 0.083 M
is 0.083 M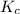
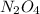 at equilibrium = 0.033 M
at equilibrium = 0.033 M
![K_c=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0600/8631/271f5.png)