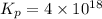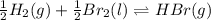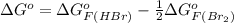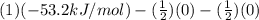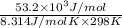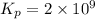Chemistry, 15.04.2020 02:33 karamalqussiri478
Be sure to answer all parts. Be sure to report your answer to the correct number of significant figures. Calculate ΔG o and KP for the following processes at 25°C: (a) H2(g) + Br2(l) ⇌ 2HBr(g) ΔG o = kJ/mol KP = × 10 (Enter your answer in scientific notation.) (b) 1 2 H2(g) + 1 2 Br2(l) ⇌ HBr(g) ΔG o = kJ/mol KP = × 10 (Enter your answer in scientific notation.)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which uses electromagnetic radiation to discover the properties and composition of bodies in space? space probe space station space shuttle space observatory
Answers: 2

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1
You know the right answer?
Be sure to answer all parts. Be sure to report your answer to the correct number of significant figu...
Questions



Mathematics, 23.07.2019 11:30





Biology, 23.07.2019 11:30


Physics, 23.07.2019 11:30

Biology, 23.07.2019 11:30

Social Studies, 23.07.2019 11:30





English, 23.07.2019 11:30


Health, 23.07.2019 11:30

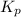 is
is 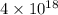 and value of
and value of 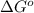 is -106.4 kJ/mol.
is -106.4 kJ/mol.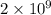 and value of
and value of 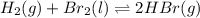
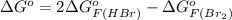
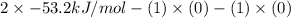
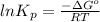
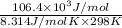
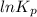 = 42.9
= 42.9