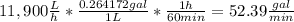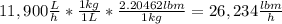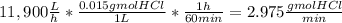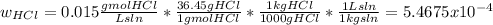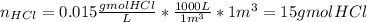The average flowrate of hydrochloric acid solution from a plant is 11,900 Lt/hr. The density is the same as that of water (1kg/Lt). The concentration of HCl in the solution is 0.015gmol/Lt.
Calculate the following:
(i) Flowrate in gallons per minute
(ii) Mass flowrate in lbm / hr
(iii) The number of gram mol of HCl flowing per minute
(iv) The mass fraction of HCl in the solution
(v) The number of lb mols of HCl in 1m3 of solution

Answers: 3
Another question on Chemistry


Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1

Chemistry, 23.06.2019 06:30
What type of chemical reaction occurs between silver nitrate (agno3) and copper (cu)? the equation i was given is 2agno3 + cu —> 2ag+ cu(no3)2.
Answers: 1

Chemistry, 23.06.2019 10:20
Determine the mass of the object below with accuracy and to the correct degree of precision. a. 324.2 g b. 324 g c. 324.30 g d. 324.25 g
Answers: 3
You know the right answer?
The average flowrate of hydrochloric acid solution from a plant is 11,900 Lt/hr. The density is the...
Questions

History, 25.10.2020 02:20

Chemistry, 25.10.2020 02:20



English, 25.10.2020 02:20



Physics, 25.10.2020 02:20

Mathematics, 25.10.2020 02:20





Geography, 25.10.2020 02:20

Mathematics, 25.10.2020 02:20

Biology, 25.10.2020 02:20

Mathematics, 25.10.2020 02:20