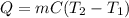In a coffee-cup calorimeter, 100.0 g of H2O and 100.0 mL of HCl are mixed. The HCl had an initial temperature of 44.6 oC and the water was originally at 24.6 oC. After the reaction, the temperature of both substances is 31.3 oC.
a. Was the reaction exothermic or endothermic?Explain.
b. Calculate how much heat the water lost or gained.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Temperature and kinetic energy are proportional. a) adirectly b) directly c) indirectly
Answers: 2

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1


Chemistry, 23.06.2019 12:30
)a children’s liquid cold medicine has a density of 1.23 g/ml. if a child is to take 2.5 tsp in a dose, what is the mass in grams of this dose? (1 tsp = 5 ml)
Answers: 1
You know the right answer?
In a coffee-cup calorimeter, 100.0 g of H2O and 100.0 mL of HCl are mixed. The HCl had an initial te...
Questions

Mathematics, 17.09.2019 05:10


Mathematics, 17.09.2019 05:10

Mathematics, 17.09.2019 05:10

Mathematics, 17.09.2019 05:10

English, 17.09.2019 05:10


Geography, 17.09.2019 05:10

Mathematics, 17.09.2019 05:10

Mathematics, 17.09.2019 05:10

Mathematics, 17.09.2019 05:10

Chemistry, 17.09.2019 05:10

History, 17.09.2019 05:10

Geography, 17.09.2019 05:10


Health, 17.09.2019 05:10

History, 17.09.2019 05:10

Business, 17.09.2019 05:10