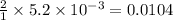A chemist prepares a solution of magnesium chloride MgCl2 by measuring out 49.mg of MgCl2 into a 100.mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of Cl−anions in the chemist's solution. Be sure your answer is rounded to the correct number of significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3
You know the right answer?
A chemist prepares a solution of magnesium chloride MgCl2 by measuring out 49.mg of MgCl2 into a 100...
Questions

History, 16.09.2019 20:50



Mathematics, 16.09.2019 20:50

History, 16.09.2019 20:50

History, 16.09.2019 20:50



Biology, 16.09.2019 20:50

Biology, 16.09.2019 20:50

Mathematics, 16.09.2019 20:50

Chemistry, 16.09.2019 20:50



Mathematics, 16.09.2019 20:50

Biology, 16.09.2019 20:50

Mathematics, 16.09.2019 20:50


English, 16.09.2019 20:50

Health, 16.09.2019 20:50

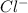 anions in the chemist's solution is 0.0104 M
anions in the chemist's solution is 0.0104 M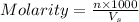
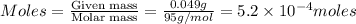
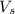 = volume of solution in ml = 100 ml
= volume of solution in ml = 100 ml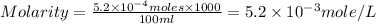
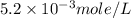
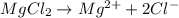
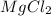 gives 2 moles of
gives 2 moles of 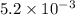 moles of
moles of