
5.00 grams of sodium hydroxide was placed in 100.0 grams of water in a coffee cup calorimeter. The temperature of the water increased from 25.0 deg C to 35.2 deg C. Find the heat of solution (ΔHsoln) for sodium hydroxide in kJ/mol. (Specific heat of water = 4.184J/g degC)?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1
You know the right answer?
5.00 grams of sodium hydroxide was placed in 100.0 grams of water in a coffee cup calorimeter. The t...
Questions

History, 17.11.2020 19:00



Social Studies, 17.11.2020 19:00


Biology, 17.11.2020 19:00

English, 17.11.2020 19:00


English, 17.11.2020 19:00

English, 17.11.2020 19:00

Mathematics, 17.11.2020 19:00

Chemistry, 17.11.2020 19:00


Mathematics, 17.11.2020 19:00

Mathematics, 17.11.2020 19:00


Social Studies, 17.11.2020 19:00



Biology, 17.11.2020 19:00

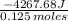
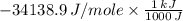 = -34.139 kJ/mol
= -34.139 kJ/mol


