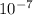Chemistry, 15.04.2020 03:41 sammysosa121832
The energy needed to ionize an atom of si when it is in the most stable is 786.4 kJ mol^-1 however if an atom of Si is in certain low lying excited state only 310.8 is needed to ionize.
what is the wavelength of he radiation emitted when an atom of si undergoes a transition from this excited state to the ground state?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
The energy needed to ionize an atom of si when it is in the most stable is 786.4 kJ mol^-1 however i...
Questions

Biology, 01.11.2020 20:10

Mathematics, 01.11.2020 20:10

Mathematics, 01.11.2020 20:10



Arts, 01.11.2020 20:10

Health, 01.11.2020 20:10




Biology, 01.11.2020 20:10

English, 01.11.2020 20:10

World Languages, 01.11.2020 20:10

Mathematics, 01.11.2020 20:10

Advanced Placement (AP), 01.11.2020 20:10

Physics, 01.11.2020 20:10


Mathematics, 01.11.2020 20:10


English, 01.11.2020 20:10

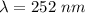
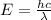
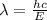 ----- (1)
----- (1)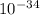 J s
J s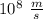
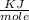
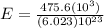
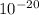 J
J
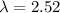 ×
×