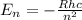Chemistry, 15.04.2020 03:00 tamekiablair502
What did Bohr conclude about the atom after observing emission spectrum lines a. Electrons can exist in any energy state but have specific states that are preferred. b. No two electrons can exist in the same quantum state within an atom. c. Possible electron energy states are quantized within an atom. d. Plum pudding tastes fantastic.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

You know the right answer?
What did Bohr conclude about the atom after observing emission spectrum lines a. Electrons can exist...
Questions

English, 22.11.2021 21:10


Chemistry, 22.11.2021 21:10


English, 22.11.2021 21:10

Social Studies, 22.11.2021 21:10


Mathematics, 22.11.2021 21:10


Medicine, 22.11.2021 21:10

Mathematics, 22.11.2021 21:10

English, 22.11.2021 21:10

Mathematics, 22.11.2021 21:10

French, 22.11.2021 21:10

Mathematics, 22.11.2021 21:10

French, 22.11.2021 21:10

Physics, 22.11.2021 21:10

English, 22.11.2021 21:10

Mathematics, 22.11.2021 21:10

Biology, 22.11.2021 21:10