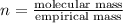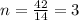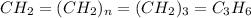Chemistry, 15.04.2020 02:55 bvbbridesmaid5519
Analysis of a sample of a gaseous compound shows that it contains 85.7% C and 14.3% H by mass. At standard conditions, 112 mL of the gaseous compound weighs 0.21 g. What is the molecular formula for the compound

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

You know the right answer?
Analysis of a sample of a gaseous compound shows that it contains 85.7% C and 14.3% H by mass. At st...
Questions

Mathematics, 14.07.2021 20:20



Business, 14.07.2021 20:20

Mathematics, 14.07.2021 20:20

Mathematics, 14.07.2021 20:20

English, 14.07.2021 20:20

Mathematics, 14.07.2021 20:20


English, 14.07.2021 20:20




History, 14.07.2021 20:20

Mathematics, 14.07.2021 20:20


English, 14.07.2021 20:20


Mathematics, 14.07.2021 20:20

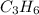
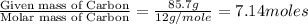
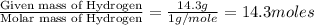
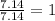
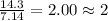
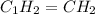
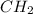 = 1(12) + 2(1) = 14 g/eq.
= 1(12) + 2(1) = 14 g/eq.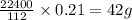 of compound
of compound