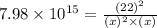Chemistry, 15.04.2020 03:10 danielburke24
The reaction SO2(g)+2H2S(g)←→3S(s)+2H2O(g) is the basis of a suggested method for removal of SO2 from power-plant stack gases. The standard free energy of each substance are ΔG∘fS(s) = 0 kJ/mol, ΔG∘fH2O(g) = -228.57 kJ/mol, ΔG∘fSO2(g) = -300.4 kJ/mol, ΔG∘fH2S(g) = -33.01 kJ/mol. If PSO2 = PH2S and the vapor pressure of water is 22 torr , calculate the equilibrium SO2 pressure in the system at 298 K.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2
You know the right answer?
The reaction SO2(g)+2H2S(g)←→3S(s)+2H2O(g) is the basis of a suggested method for removal of SO2 fro...
Questions

History, 19.10.2020 23:01


Mathematics, 19.10.2020 23:01

English, 19.10.2020 23:01







English, 19.10.2020 23:01

Social Studies, 19.10.2020 23:01


History, 19.10.2020 23:01

Mathematics, 19.10.2020 23:01

History, 19.10.2020 23:01


Mathematics, 19.10.2020 23:01

Chemistry, 19.10.2020 23:01

English, 19.10.2020 23:01

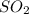 pressure is,
pressure is, 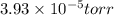
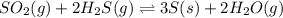
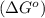 .
.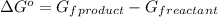
![\Delta G^o=[n_{S(s)}\times \Delta G_f^0_{(S(s))}+n_{H_2O(g)}\times \Delta G_f^0_{(H_2O(g))}]-[n_{SO_2(g)}\times \Delta G_f^0_{(SO_2(g))}+n_{H_2S(g)}\times \Delta G_f^0_{(H_2S(g))}]](/tpl/images/0600/9913/1b30c.png)
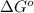 = standard free energy of reaction = ?
= standard free energy of reaction = ?![\Delta G^o=[3mole\times (0kJ/mol)+2mole\times (-228.57kJ/mol)]-[1mole\times (-300.4kJ/mol)+2mole\times (-33.01kJ/mol)]](/tpl/images/0600/9913/f2373.png)
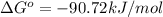
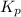
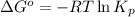
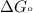 = standard Gibbs free energy = -90.72 kJ/mol
= standard Gibbs free energy = -90.72 kJ/mol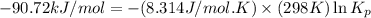
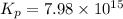
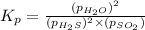
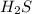 = x
= x