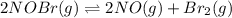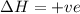Chemistry, 15.04.2020 03:13 joeykyle05
For the following reaction, the equilibrium constant Kc is 2.0 at a certain temperature. The reaction is endothermic. What do you expect to happen to the concentration of NO if the temperature is doubled? 2NOBr(g) ⇌ 2NO(g) + Br2(g)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1

Chemistry, 23.06.2019 04:00
Which method would be best to separate a mixture of sand and gravel
Answers: 1

Chemistry, 23.06.2019 06:20
An object of mass 10.0 kg and volume 1000 ml and density 10 g/ml sinks in water who’s density is 1.0 g/ml. what is the mass of the water which has been displaced in kilograms
Answers: 1
You know the right answer?
For the following reaction, the equilibrium constant Kc is 2.0 at a certain temperature. The reactio...
Questions

History, 23.05.2021 14:00


Social Studies, 23.05.2021 14:00

English, 23.05.2021 14:00

English, 23.05.2021 14:00

Mathematics, 23.05.2021 14:00

Chemistry, 23.05.2021 14:00

Mathematics, 23.05.2021 14:00

Geography, 23.05.2021 14:00


Mathematics, 23.05.2021 14:00

History, 23.05.2021 14:00


History, 23.05.2021 14:00



English, 23.05.2021 14:00

Engineering, 23.05.2021 14:00

Mathematics, 23.05.2021 14:00

Computers and Technology, 23.05.2021 14:00