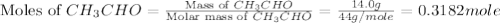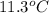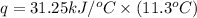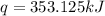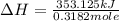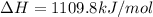Chemistry, 15.04.2020 03:33 tusharchandler124
The heat capacity of a bomb calorimeter was determined by burning 6.91 g of methane (energy of combustion = −803 kJ/mol CH4) in the bomb. The temperature changed by 11.1°C. (a) What is the heat capacity of the bomb? kJ/°C (b) A 14.0-g sample of acetaldehyde (CH3CHO) produced a temperature increase of 11.3°C in the same calorimeter. What is the energy of combustion of acetaldehyde (in kJ/mol)?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3
You know the right answer?
The heat capacity of a bomb calorimeter was determined by burning 6.91 g of methane (energy of combu...
Questions

Arts, 01.09.2020 05:01

Biology, 01.09.2020 05:01



Mathematics, 01.09.2020 05:01

Mathematics, 01.09.2020 05:01

Advanced Placement (AP), 01.09.2020 05:01


English, 01.09.2020 05:01

Mathematics, 01.09.2020 05:01


Mathematics, 01.09.2020 05:01

Computers and Technology, 01.09.2020 05:01

Mathematics, 01.09.2020 05:01

Mathematics, 01.09.2020 05:01


Mathematics, 01.09.2020 05:01

Mathematics, 01.09.2020 05:01

Chemistry, 01.09.2020 05:01

Mathematics, 01.09.2020 05:01

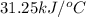
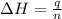
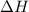 = enthalpy change = -803 kJ/mol
= enthalpy change = -803 kJ/mol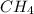 = 6.91 g
= 6.91 g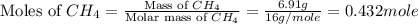
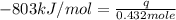
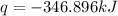
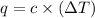
 = change in temperature =
= change in temperature = 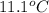
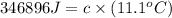
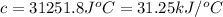
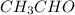 = 14.0 g
= 14.0 g