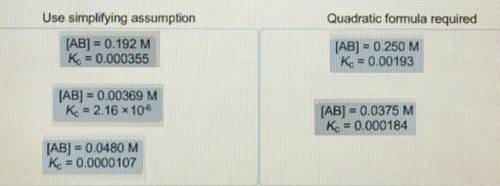
When determining the equilibrium concentrations for the reaction ; a simplifying assumption can be used under certain conditions to avoid solving a quadratic equation. Classify each situation by whether the simplifying assumption can be used or whether the quadratic formula is required. Use simplifying assumptions - Quadratic formula required 1. [AB] = 0.0178 M; 2. [AB] = 0.00204 M; 3. [AB] =0.451 M; = 0.000905 4. [AB] = 0.0174 M; = 0.0000925 5. [AB] = 0.396 M; = 0.00228

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2
You know the right answer?
When determining the equilibrium concentrations for the reaction ; a simplifying assumption can be...
Questions


World Languages, 21.02.2021 14:00

Mathematics, 21.02.2021 14:00



History, 21.02.2021 14:00

Mathematics, 21.02.2021 14:00

Mathematics, 21.02.2021 14:00

Mathematics, 21.02.2021 14:00


English, 21.02.2021 14:00




Physics, 21.02.2021 14:00


Social Studies, 21.02.2021 14:00

Mathematics, 21.02.2021 14:00


Mathematics, 21.02.2021 14:00