
Carbon's valence of four most directly results from its a. tetrahedral shape. b. four electrons in the valence shell that can form four covalent bonds. c. ability to form single, double, and triple bonds. d. ability to form chains and rings of carbon atoms. 2. Hydrocarbons are not soluble in water because a. they are hydrophilic. b. their C-H bonds are nonpolar. c. they do not ionize. d. they are lighter than water. 3. Which of the following is not true of an asymmet- ric carbon atom

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1
You know the right answer?
Carbon's valence of four most directly results from its a. tetrahedral shape. b. four electrons in t...
Questions


Biology, 05.08.2019 01:00


History, 05.08.2019 01:00

Biology, 05.08.2019 01:00



Physics, 05.08.2019 01:00


Physics, 05.08.2019 01:00


Mathematics, 05.08.2019 01:00




Biology, 05.08.2019 01:00

History, 05.08.2019 01:00

History, 05.08.2019 01:00

Biology, 05.08.2019 01:00

History, 05.08.2019 01:00

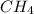 is a hydrocarbon.
is a hydrocarbon.

