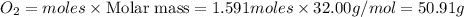Chemistry, 15.04.2020 03:54 Ashley606hernandez
Diborane (B2H6) is a gas at room temperature that forms explosive mixtures with air. It reacts with oxygen according to the following equation (which may or may not be balanced): B2H6 (g) + O2 (g) → B2O3 (s) + H2O (l) How many grams of O2 (molar mass 32.00 g/mol) will react with 14.67 grams of diborane (molar mass 27.67 g/mol). Your answer must be expressed to the correct number of significant figures, and with the correct unit.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

Chemistry, 23.06.2019 05:50
Which of the following is not a characteristic of s waves?
Answers: 1


Chemistry, 23.06.2019 14:00
How many moles of oxygens atoms are present in 5.00 mol of diphosphorus of fe2(so4)3
Answers: 2
You know the right answer?
Diborane (B2H6) is a gas at room temperature that forms explosive mixtures with air. It reacts with...
Questions

History, 18.03.2021 01:50

Mathematics, 18.03.2021 01:50

Biology, 18.03.2021 01:50


Biology, 18.03.2021 01:50


Health, 18.03.2021 01:50


Mathematics, 18.03.2021 01:50

Business, 18.03.2021 01:50


Mathematics, 18.03.2021 01:50

Mathematics, 18.03.2021 01:50


Mathematics, 18.03.2021 01:50

Arts, 18.03.2021 01:50

Mathematics, 18.03.2021 01:50


Biology, 18.03.2021 01:50

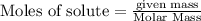
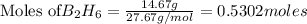
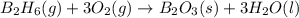
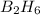 require = 3 moles of
require = 3 moles of 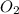
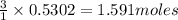 of
of