
Chemistry, 15.04.2020 04:41 Madisonk3571
A hydrated form of copper sulfate ( CuSO 4 ⋅ x H 2 O ) is heated to drive off all of the water. If there is initially 7.74 g of hydrated salt and there is 4.95 g of anhydrous CuSO 4 after heating, find the number of water molecules associated with each CuSO 4 formula unit.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2

Chemistry, 22.06.2019 06:10
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3
You know the right answer?
A hydrated form of copper sulfate ( CuSO 4 ⋅ x H 2 O ) is heated to drive off all of the water. If t...
Questions

Mathematics, 26.10.2021 08:00

Mathematics, 26.10.2021 08:00



Physics, 26.10.2021 08:00



Mathematics, 26.10.2021 08:00




Mathematics, 26.10.2021 08:00

Mathematics, 26.10.2021 08:00

History, 26.10.2021 08:00

Mathematics, 26.10.2021 08:00



Mathematics, 26.10.2021 08:00

History, 26.10.2021 08:00

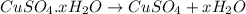
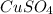 = 159.6 g/mol
= 159.6 g/mol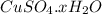 decomposes to give = 159.6g of
decomposes to give = 159.6g of  g
g


