
Chemistry, 15.04.2020 04:31 hdhdhd49jdhd
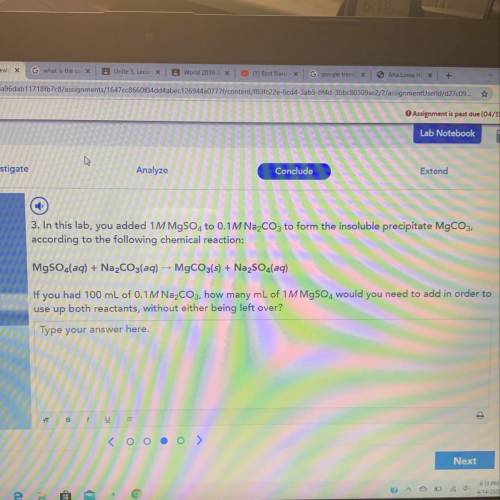
If u had 100mL of 0.1M Na2CO3 how many ML of 1M MgSO4 would you need to add in order to use up both reactants without either being left over

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:10
Seawater contains approximately 3.5%nacl by mass and has a density of 1.02 g/ml. what volume of seawater contains 7.5 g of sodium?
Answers: 2

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

You know the right answer?
If u had 100mL of 0.1M Na2CO3 how many ML of 1M MgSO4 would you need to add in order to use up both...
Questions

Mathematics, 20.01.2021 17:40




Mathematics, 20.01.2021 17:40

Mathematics, 20.01.2021 17:40


Mathematics, 20.01.2021 17:40

History, 20.01.2021 17:40


Physics, 20.01.2021 17:40

Mathematics, 20.01.2021 17:40


History, 20.01.2021 17:40

Social Studies, 20.01.2021 17:40


Mathematics, 20.01.2021 17:40

English, 20.01.2021 17:40

Mathematics, 20.01.2021 17:40

Mathematics, 20.01.2021 17:40



