Chemistry, 15.04.2020 04:48 anitaabbey27
Linolenic acid (C18H30O2) can be hydrogenated to stearic acid by reacting it with hydrogen gas according to the equation: C18H30O2 + 3H2 --->C18H36O2 What volume of hydrogen gas, measured at STP, is required to react with 10.5 g of linolenic acid in this reaction?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1

Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
You know the right answer?
Linolenic acid (C18H30O2) can be hydrogenated to stearic acid by reacting it with hydrogen gas accor...
Questions

Social Studies, 18.05.2021 17:30


Mathematics, 18.05.2021 17:30



Mathematics, 18.05.2021 17:30

English, 18.05.2021 17:30

Mathematics, 18.05.2021 17:30


Computers and Technology, 18.05.2021 17:30

Mathematics, 18.05.2021 17:30

Physics, 18.05.2021 17:30

Social Studies, 18.05.2021 17:30

Mathematics, 18.05.2021 17:30



English, 18.05.2021 17:30

Social Studies, 18.05.2021 17:30


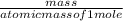
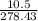
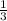 =
=