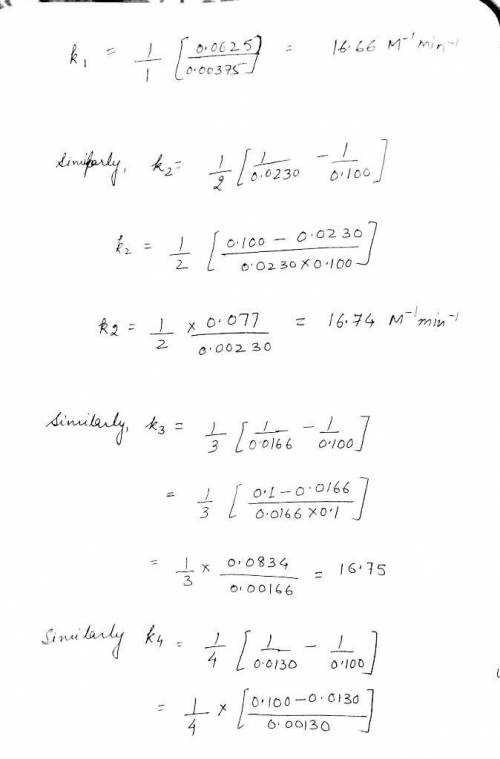2NH3g+N2g3H2g

Chemistry, 15.04.2020 16:09 aleexandras09
A chemistry graduate student is studying the rate of this reaction:
2NH3g+N2g3H2g
He fills a reaction vessel with NH3 and measures its concentration as the reaction proceeds:
time (minutes) NH3 0 0.100M 1.0 0.0375M 2.0 0.0230M 3.0 0.0166M 4.0 0.0130M
Use this data to answer the following questions.
Write the rate law for this reaction. rate =k NH3
Calculate the value of the rate constant k . Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. =k ×10

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1


Chemistry, 23.06.2019 02:00
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1

You know the right answer?
A chemistry graduate student is studying the rate of this reaction:
2NH3g+N2g3H2g
2NH3g+N2g3H2g
Questions


Mathematics, 13.10.2019 17:30

Mathematics, 13.10.2019 17:30







Mathematics, 13.10.2019 17:30


Health, 13.10.2019 17:30

Mathematics, 13.10.2019 17:30


Mathematics, 13.10.2019 17:30


English, 13.10.2019 17:30


History, 13.10.2019 17:30