
Chemistry, 15.04.2020 17:31 cheyennemitchel2680
You will often work with salts of , , and in the laboratory. (All are found in nature, and all are important economically.) If you have a solution containing these three ions, each at a concentration of 0.10 M, what is the order in which their hydroxides begin to precipitate as aqueous NaOH is slowly added to the solution? Formula Enter an integer to indicate order: 1 = first, 2 = second, 3 = last : : :

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1
You know the right answer?
You will often work with salts of , , and in the laboratory. (All are found in nature, and all are i...
Questions


Mathematics, 29.01.2020 05:00



History, 29.01.2020 05:00


Mathematics, 29.01.2020 05:00



Spanish, 29.01.2020 05:00

Biology, 29.01.2020 05:00

Mathematics, 29.01.2020 05:00


English, 29.01.2020 05:00

Mathematics, 29.01.2020 05:00

Mathematics, 29.01.2020 05:00

Mathematics, 29.01.2020 05:00

Social Studies, 29.01.2020 05:00

Mathematics, 29.01.2020 05:00

History, 29.01.2020 05:00

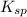 values of each individual precipitate. The lower the
values of each individual precipitate. The lower the 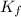 = 1.1 x 10³³)
= 1.1 x 10³³)

