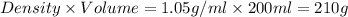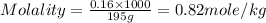Chemistry, 15.04.2020 18:55 drelisech6467
A student dissolves of 15 g aniline in of a solvent with a density of . The student notices that the volume of the solvent does not change when the aniline dissolves in it. Calculate the molarity and molality of the student's solution. Be sure each of your answer entries has the correct number of significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

Chemistry, 23.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 4.20 mol fe and 6.70 mol nio(oh) react?
Answers: 3

Chemistry, 23.06.2019 22:00
Calculate the partial pressure, in atmospheres, of o2 in the dry air outside an airliner cruising at an altitude of about 20000 ft (6096 m), where the atmoshperic pressure is 351 mm hg. how much must the outside air be compressed to produce a cabin pressure in which the partial pressure of o2 is 0.200 atm?
Answers: 2

Chemistry, 23.06.2019 22:50
Which of the following is the best explanation why some reactions appear to stop before all the reactants are converted to products? the reaction goes to completion. the reaction reaches equilibrium and the products stop being formed. the reversible reaction occurs at the same rate. the limiting reactant prevents the forward reaction occurring.
Answers: 1
You know the right answer?
A student dissolves of 15 g aniline in of a solvent with a density of . The student notices that the...
Questions




Mathematics, 16.07.2021 20:40





Mathematics, 16.07.2021 20:40



Computers and Technology, 16.07.2021 20:50








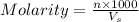
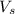 = volume of solution in ml = 200 ml
= volume of solution in ml = 200 ml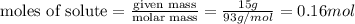
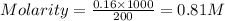
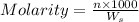
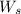 = weight of solvent in g
= weight of solvent in g