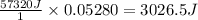A student enters the lab and conducts Part A of the Experiment. The student uses 25.00 mL of 2.112 M HCl, and adds NaOH in excess as instructed. If the ΔH of the neutralization reaction is known to be -57,320 J/mol H2O, what is the total theoretical heat released (in Joules)?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3
You know the right answer?
A student enters the lab and conducts Part A of the Experiment. The student uses 25.00 mL of 2.112 M...
Questions

English, 05.10.2021 22:10

History, 05.10.2021 22:10

Health, 05.10.2021 22:10

History, 05.10.2021 22:10



Social Studies, 05.10.2021 22:10

Computers and Technology, 05.10.2021 22:10




German, 05.10.2021 22:10

Health, 05.10.2021 22:10

Geography, 05.10.2021 22:10

Biology, 05.10.2021 22:10


Mathematics, 05.10.2021 22:10



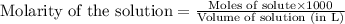 .....(1)
.....(1)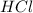 solution = 2.112 M
solution = 2.112 M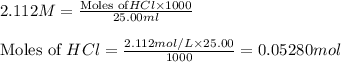

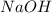 is the excess reagent.
is the excess reagent.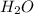
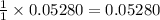 moles of
moles of