
Chemistry, 15.04.2020 18:26 FailingstudentXD
The reaction of Fe3O4(s) with hydrogen(g) to form iron(s) and water(g) proceeds as follows: Fe3O4(s) + 4 H2(g) 3 Fe(s) + 4 H2O(g) When 61.8 grams of Fe3O4(s) react with sufficient H2(g) , 40.3 kJ of energy are absorbed . What is the value of H for the chemical equation given?

Answers: 2
Another question on Chemistry

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3

Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2


Chemistry, 23.06.2019 06:00
Nthis lab, you will do experiments to identify types of changes. using the question format you learned (shown above), write an investigative question that you can answer by doing these experiments
Answers: 3
You know the right answer?
The reaction of Fe3O4(s) with hydrogen(g) to form iron(s) and water(g) proceeds as follows: Fe3O4(s)...
Questions

English, 19.03.2020 21:36




English, 19.03.2020 21:36

English, 19.03.2020 21:36

Social Studies, 19.03.2020 21:36


Health, 19.03.2020 21:36



Mathematics, 19.03.2020 21:37

Mathematics, 19.03.2020 21:37




History, 19.03.2020 21:37


Computers and Technology, 19.03.2020 21:37

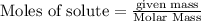
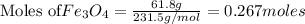
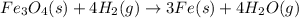
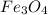 absorb energy = 40.3 kJ
absorb energy = 40.3 kJ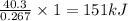
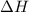 for the chemical equation given is 151 kJ
for the chemical equation given is 151 kJ

