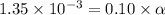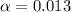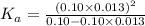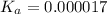Chemistry, 15.04.2020 20:21 tiffanybrandy23
Determine the acid dissociation constant for a 0.10 m acetic acid solution that has a ph of 2.87. Acetic acid is a weak monoprotic acid and the equilibrium equation of interest is

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2


Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1
You know the right answer?
Determine the acid dissociation constant for a 0.10 m acetic acid solution that has a ph of 2.87. Ac...
Questions


Mathematics, 23.05.2020 17:57


Computers and Technology, 23.05.2020 17:57

Mathematics, 23.05.2020 17:57

Social Studies, 23.05.2020 17:57

Mathematics, 23.05.2020 17:57

Mathematics, 23.05.2020 17:57


Mathematics, 23.05.2020 17:57



Mathematics, 23.05.2020 17:57



Mathematics, 23.05.2020 17:57


Mathematics, 23.05.2020 17:57


Mathematics, 23.05.2020 17:57

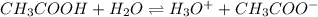
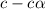
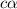
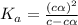
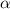 = dissociation constant = ?
= dissociation constant = ?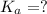
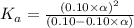
![pH=-log[H^+]](/tpl/images/0602/6192/15713.png)
![2.87=-log[H^+]](/tpl/images/0602/6192/3a07c.png)
![[H^+]=1.35\times 10^{-3}](/tpl/images/0602/6192/8f9f0.png)
![[H^+]=c\times \alpha](/tpl/images/0602/6192/4fc41.png)