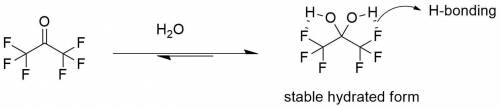
For most ketones, hydrate formation is unfavorable, because the equilibrium favors the ketone rather than the hydrate. However, the equilibrium for hydration of hexafluoroacetone favors formation of the hydrate. Provide a plausible explanation for this observation.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2
You know the right answer?
For most ketones, hydrate formation is unfavorable, because the equilibrium favors the ketone rather...
Questions


History, 30.08.2019 00:30

History, 30.08.2019 00:30





History, 30.08.2019 00:30




Social Studies, 30.08.2019 00:30

History, 30.08.2019 00:30


Chemistry, 30.08.2019 00:30

Mathematics, 30.08.2019 00:30

Mathematics, 30.08.2019 00:30

History, 30.08.2019 00:30


Mathematics, 30.08.2019 00:30

 can not escape the hydrated form and goes back to keto form.
can not escape the hydrated form and goes back to keto form.