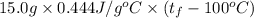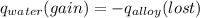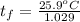Chemistry, 15.04.2020 20:48 jforeman42
A 15.0 g sample of nickel metal is heated to 100.0 degrees C and dropped into 55.0 g of water, initially at 23.0 degrees C. Assuming that all the heat lost by nickel is absorbed by the water, calculate the final temperature of the nickel and water. (C

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Put these processes of the water cycle in the correct order, starting at the point where the water is in the lake: 1. water evaporates into the atmosphere 2. rain, snow, or other precipitation falls 3. water collects into larger bodies of water 4. water vapor condenses into liquid water
Answers: 1

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3
You know the right answer?
A 15.0 g sample of nickel metal is heated to 100.0 degrees C and dropped into 55.0 g of water, initi...
Questions





Spanish, 12.02.2020 07:13



Mathematics, 12.02.2020 07:13

Mathematics, 12.02.2020 07:13

Mathematics, 12.02.2020 07:13

Mathematics, 12.02.2020 07:13

Mathematics, 12.02.2020 07:13


Mathematics, 12.02.2020 07:13


Medicine, 12.02.2020 07:13


Mathematics, 12.02.2020 07:13


 .
.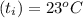 ,
, 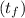 = ?,
= ?,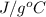 ,
, 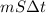
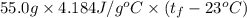
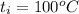 ,
,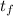 = ?
= ?