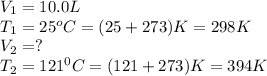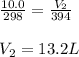Chemistry, 15.04.2020 20:57 blakesmith0110
A fixed amount of gas at 25.0 °C occupies a volume of 10.0 L when the pressure is 629 torr. Using Charles's law to calculate the volume (L) the gas will occupy when the temperature is increased 121 °C while maintaining the pressure at 629 torr.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1
You know the right answer?
A fixed amount of gas at 25.0 °C occupies a volume of 10.0 L when the pressure is 629 torr. Using Ch...
Questions











Mathematics, 14.04.2020 04:49





Mathematics, 14.04.2020 04:49


Biology, 14.04.2020 04:49


Mathematics, 14.04.2020 04:49

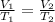
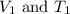 are the initial volume and temperature of the gas.
are the initial volume and temperature of the gas.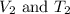 are the final volume and temperature of the gas.
are the final volume and temperature of the gas.