
Chemistry, 15.04.2020 20:56 harodkdc7910
Suppose you store a 15.5 g piece of aluminum (Cp of Al = 0.902 J/g⁰C) in the refrigerator at 2.3⁰C and then drop it into your coffee. The coffee temperature drops from 95.6⁰C to 45.6⁰C. How many Joules of energy did the aluminum block absorb?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1

Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1

You know the right answer?
Suppose you store a 15.5 g piece of aluminum (Cp of Al = 0.902 J/g⁰C) in the refrigerator at 2.3⁰C a...
Questions



Mathematics, 05.06.2021 19:10






Mathematics, 05.06.2021 19:10

Mathematics, 05.06.2021 19:10

Biology, 05.06.2021 19:10

History, 05.06.2021 19:10


Social Studies, 05.06.2021 19:10

Law, 05.06.2021 19:10


History, 05.06.2021 19:10

English, 05.06.2021 19:10


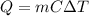
 is the change in temperature
is the change in temperature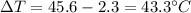 is the change in temperature of the aluminium (in fact, at thermal equilibrium, the block of aluminium reaches the same final temperature as the coffee)
is the change in temperature of the aluminium (in fact, at thermal equilibrium, the block of aluminium reaches the same final temperature as the coffee)


