Chemistry, 15.04.2020 21:29 gutuerrezsebastian
If a 95.0 gram sample of metal at 100.0 oC is placed in 50.0 g of water with an initial temperature of 22.5oC and the final temperature of the system is 48.5oC, what is the specific heat of the metal? Group of answer choices

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2
You know the right answer?
If a 95.0 gram sample of metal at 100.0 oC is placed in 50.0 g of water with an initial temperature...
Questions






Mathematics, 07.07.2019 15:00


Mathematics, 07.07.2019 15:00






Mathematics, 07.07.2019 15:00


Mathematics, 07.07.2019 15:00

Physics, 07.07.2019 15:00


History, 07.07.2019 15:00

Computers and Technology, 07.07.2019 15:00

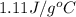
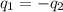
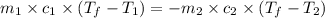
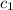 = specific heat of metal = ?
= specific heat of metal = ?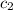 = specific heat of water =
= specific heat of water = 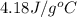
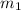 = mass of metal = 95.0g
= mass of metal = 95.0g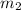 = mass of water = 50.0 g
= mass of water = 50.0 g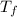 = final temperature of mixture =
= final temperature of mixture = 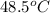
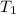 = initial temperature of metal =
= initial temperature of metal = 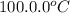
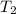 = initial temperature of water =
= initial temperature of water = 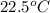
![(95.0g)\times c_1\times (48.5-100.0)^oC=-[(50.0g)\times 4.18J/g^oC\times (48.5-22.5)^oC]](/tpl/images/0602/9464/68c4b.png)