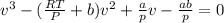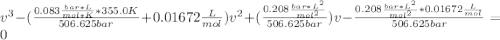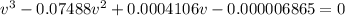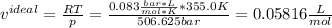Use the van der Waals equation and the ideal gas equation to calculate the volume of 1.000 mol of neon at a pressure of 500.0 atm and a temperature of 355.0 K. Explain why the two values are different. (Hint: One way to solve the van der Waals equation for V is to use successive approximations. Use the ideal gas law to get a preliminary estimate for V.)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
The wave shown on the electromagnetic spectrum disturb the medium it passes through a)different frequency. b)the same frequency .
Answers: 2

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
Use the van der Waals equation and the ideal gas equation to calculate the volume of 1.000 mol of ne...
Questions

Mathematics, 14.04.2021 23:00


Chemistry, 14.04.2021 23:00

Physics, 14.04.2021 23:00

Mathematics, 14.04.2021 23:00

Mathematics, 14.04.2021 23:00


English, 14.04.2021 23:00

Social Studies, 14.04.2021 23:00



English, 14.04.2021 23:00


English, 14.04.2021 23:00


Mathematics, 14.04.2021 23:00

Mathematics, 14.04.2021 23:00

Mathematics, 14.04.2021 23:00


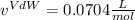
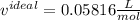
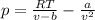
 and
and 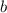 are referred to the attraction and repulsion effect, in such a way, for neon, such values are 0.208 bar*L²/mol² and 0.01672 L/mol respectively. However, with the given information, the polynomic form of the VdW equation is:
are referred to the attraction and repulsion effect, in such a way, for neon, such values are 0.208 bar*L²/mol² and 0.01672 L/mol respectively. However, with the given information, the polynomic form of the VdW equation is: