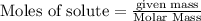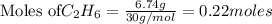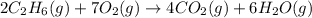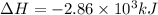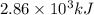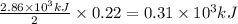Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:00
If a planet rotates 360 degrees during a 24 hour time period, what does that tell us about the planet? a. the middle of the planet is in darkness b. the seasons on the planet vary every day. c. the planet runs on a 12-hour time clock. d. the temperature on the planet varies daily.
Answers: 1


Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1
You know the right answer?
The following thermochemical equation is for the reaction of ethane(g) with oxygen(g) to form carbon...
Questions



History, 22.10.2020 21:01





Mathematics, 22.10.2020 21:01



Mathematics, 22.10.2020 21:01

History, 22.10.2020 21:01



English, 22.10.2020 21:01

Mathematics, 22.10.2020 21:01



Mathematics, 22.10.2020 21:01

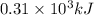 of energy is produced
of energy is produced