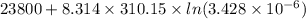The standard free-energy change for this reaction in the direction written is 23.8 kJ/mol. The concentrationsof the three intermediates in the hepatocyte of a mammal are: fructose 1,6-bisphosphate,1.4X10-5 M; glyceraldehyde 3-phosphate, 3X10-6 M; and dihydroxyacetone phosphate, 1.6X10-5 M. At body temperature (37C), what is the actual free-energy change for the reaction

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1
You know the right answer?
The standard free-energy change for this reaction in the direction written is 23.8 kJ/mol. The conce...
Questions


Chemistry, 17.10.2019 18:30

English, 17.10.2019 18:30




History, 17.10.2019 18:30

English, 17.10.2019 18:30

History, 17.10.2019 18:30


Chemistry, 17.10.2019 18:30





Social Studies, 17.10.2019 18:30

Mathematics, 17.10.2019 18:30

Computers and Technology, 17.10.2019 18:30


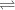 Glyceraldehyde 3-phosphate + DHAP
Glyceraldehyde 3-phosphate + DHAP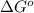 is 23.8 kJ/mol.
is 23.8 kJ/mol. 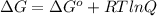
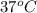
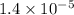 M
M
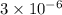 M
M
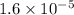 M
M ![\frac{[DHAP][\text{glyceraldehyde 3-phosphate}]}{[/text{Fructose 1,6-bisphosphate}]}](/tpl/images/0603/3958/6ad8b.png)
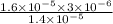
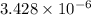
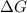 as follows.
as follows.