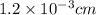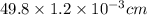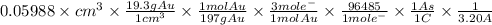Chemistry, 15.04.2020 23:16 lilsnsbsbs
The surface area of an object to be gold plated is 49.8 cm2 and the density of gold is 19.3 g/cm-3. A current of 3.15. A is applied to a solution that contains gold in the +3 oxidation state. Calculate the time required to deposit an even layer of gold 1.2 x 10 -3cm thick on the object.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3
You know the right answer?
The surface area of an object to be gold plated is 49.8 cm2 and the density of gold is 19.3 g/cm-3....
Questions







Mathematics, 28.02.2020 23:52

Mathematics, 28.02.2020 23:52





Mathematics, 28.02.2020 23:53

Physics, 28.02.2020 23:53





Mathematics, 28.02.2020 23:53

 sec.
sec.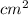 ,
,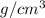 ,
,