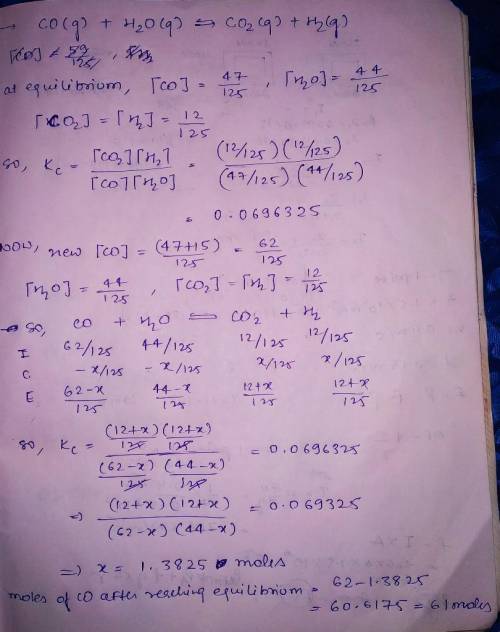Chemistry, 15.04.2020 23:28 mathscience9301
"Synthesis gas" is a mixture of carbon monoxide and water vapor. At high temperature synthesis gas will form carbon dioxide and hydrogen, and in fact this reaction is one of the ways hydrogen is made industrially. A chemical engineer studying this reaction fills a tank with of carbon monoxide gas and of water vapor. When the mixture has come to equilibrium he determines that it contains of carbon monoxide gas, of water vapor and of carbon dioxide. The engineer then adds another of carbon monoxide, and allows the mixture to come to equilibrium again. Calculate the moles of after equilibrium is reached the second time. Round your answer to significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 12:30
Activity two: just lemons, inc. production here's a one-batch sample of just lemons lemonade production. determine the percent yield and amount of leftover ingredients for lemonade production and place your answers in the data chart. hint: complete stoichiometry calculations for each ingredient to determine the theoretical yield. complete a limiting reactant-to-excess reactant calculation for both excess ingredients. water sugar lemon juice lemonade percent yield leftover ingredients 946.36 g 196.86 g 193.37 g 2050.25 g 89% just lemons lemonade recipe equation: 2 water + sugar + lemon juice = 4 lemonade mole conversion factors: 1 mole of water = 1 cup = 236.59 g 1 mole of sugar = 1 cup = 225 g 1 mole of lemon juice = 1 cup = 257.83 g 1 mole of lemonade = 1 cup = 719.42 g show your calculations below. analysis questions 1. based on taste observations only, which ingredients were in excess in the lemonade samples in activity one? in activity one the excess substances for each sample were the water and sugar. 2. based on the data in activity two, which excess ingredients are affecting the taste of the lemonade in the sample batch? 3. what can just lemons, inc. do during production to reduce the amount of excess ingredients and improve the taste of their lemonade? 4. try to reduce the amount of leftover ingredients by changing the amount of one, two, or all three starting ingredients. show your stoichiometric calculations below. 5. during factory inspection, just lemons, inc. discovered that a water valve to the lemonade mixing station was not functioning. once they repair it, more water will enter the mixing station. from what you know about the limiting and excess ingredients for current lemonade production, what advice would you give engineers about the upcoming increase in water?
Answers: 1



You know the right answer?
"Synthesis gas" is a mixture of carbon monoxide and water vapor. At high temperature synthesis gas w...
Questions



Mathematics, 03.09.2020 05:01

Mathematics, 03.09.2020 05:01

History, 03.09.2020 05:01

Mathematics, 03.09.2020 05:01

Physics, 03.09.2020 05:01

Biology, 03.09.2020 05:01

Mathematics, 03.09.2020 05:01

Mathematics, 03.09.2020 05:01

Mathematics, 03.09.2020 05:01


History, 03.09.2020 05:01




Mathematics, 03.09.2020 05:01

Mathematics, 03.09.2020 05:01


Mathematics, 03.09.2020 05:01