Chemistry, 16.04.2020 00:23 lerasteidl
Measurements show that the enthalpy of a mixture of gaseous reactants increases by 243.kJ during a certain chemical reaction, which is carried out at a constant pressure. Furthermore, by carefully monitoring the volume change it is determined that −174.kJ of work is done on the mixture during the reaction. Calculate the change of energy of the gas mixture during the reaction in kJ.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1


Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1
You know the right answer?
Measurements show that the enthalpy of a mixture of gaseous reactants increases by 243.kJ during a c...
Questions



Mathematics, 30.07.2020 06:01

Mathematics, 30.07.2020 06:01



Mathematics, 30.07.2020 06:01



English, 30.07.2020 06:01


Mathematics, 30.07.2020 06:01






English, 30.07.2020 06:01


Mathematics, 30.07.2020 06:01

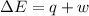
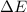 =Change in internal energy
=Change in internal energy