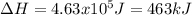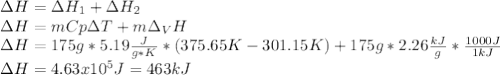Salt water can be desalinated by distillation. How much energy is needed to convert 175 g of salt water at 28.0 °C to water vapor if the specific heat of salt water is 5.19 J/g K, the boiling point of salt water is 102.5 °C, and the enthalpy of vaporization is 2.26 kJ/g?
A. 317 kJ
B. 399 kJ
C. 463 kJ
D. 512 kJ
E. 673 kJ

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3


Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 23.06.2019 01:00
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3
You know the right answer?
Salt water can be desalinated by distillation. How much energy is needed to convert 175 g of salt wa...
Questions
















Computers and Technology, 28.01.2020 01:31



Computers and Technology, 28.01.2020 01:31